Figure 2.1 shows a diagram of the main
feedforward pathway in the visual system of humans
(see Wandell 1995 and Kandel et al. 1991 for
an overview).
There is a consensus that significant information processing occurs in the retina and V1 (Kandel et al., 1991). Although they are often modeled as simple filters, the earliest stages of visual perception are perhaps better described by a feature detector model (Hubel and Wiesel 1959, 1965; Van Essen 1992). Neurons at each level detect certain features, sending their outputs to higher levels for the detection of progressively more complex stimuli.
For instance, retinal ganglion cells in the eye typically respond most strongly to a circular light or dark spot in a particular area of the retina (its receptive field) (Dacey, 1994; Kaplan, 1989). Neurons in the LGN behave similarly (Casagrande and Norton, 1989). Beginning in the primary visual cortex, most neurons are found instead to prefer inputs elongated in some particular direction (Hubel and Wiesel, 1968). Such neurons respond most strongly to an oriented stimulus such as a line or an edge close to a preferred orientation. They respond less strongly the more the orientation differs from the preference, do not generally respond to unoriented inputs, and respond equally well over a wide range of contrasts. Thus V1 neurons are considered detectors for their preferred orientation, and neural explanations of orientation perception usually begin at this level of processing. Higher visual processing areas in the brain all use the output from this stage, eventually detecting more complex features such as human faces (Van Essen, 1992).
The primary visual cortex, like the other parts of the cortex, is composed of a two-dimensional, slightly folded sheet of neurons and connections between them. If flattened, human V1 would cover an area of nearly four square inches (Wandell, 1995). It contains at least 150 million neurons, each making many hundreds of specific connections with other neurons (Wandell, 1995). The neurons are arranged in six layers with different anatomical characteristics (using Brodmann's scheme for numbering laminations in human V1; see Henry 1989 for more details). Input from the thalamus (the afferent input) typically goes to layer 4 (Casagrande and Norton, 1989; Henry, 1989). Neurons in the other layers form local connections within V1 or connect to other visual processing areas such as V2 (adjacent to V1).
At a given location on the cortical sheet, the neurons in a vertical section through the cortex generally have the same preference for the eye of origin, stimulus orientation, stimulus size, etc. It is customary to refer to such a section as a column (Gilbert and Wiesel, 1989). The RF-LISSOM model discussed in this thesis will treat each column as a single unit, thus representing the cortex as a purely two-dimensional surface. This is only an approximation, but it is a valuable one because it greatly simplifies the analysis while retaining the basic functional features of the cortex.
Nearby columns generally have similar, but not identical, preferences, and slightly more distant columns generally have more dissimilar preferences. Preferences repeat at regular intervals (approximately 1-2 mm) in every direction. For orientation preferences, this arrangement of detectors forms a smoothly varying orientation map of the retinal input (Blasdel and Salama, 1986; Blasdel, 1992a; Grinvald et al., 1994; Ts'o et al., 1990; Weliky and Kandler, 1995). (See figure 4.3b in chapter 4 for an example of an orientation map.) Each location on the retina is mapped to a region on the orientation map, with each possible orientation at that location represented by different orientation detectors. The global layout of the orientation map, and consequently the orientation preferences of the individual neurons in the map, is formed during the early development of the animal based on its visual experience (Blakemore and Cooper 1970; Blakemore and van Sluyters 1975; Hubel and Wiesel 1962, 1968; Movshon and van Sluyters 1981).
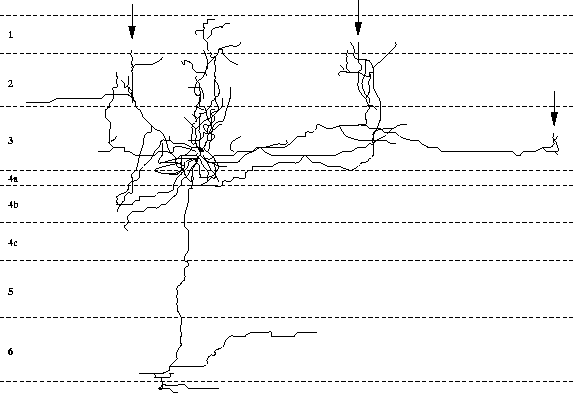
Extensive, long-range lateral connections are present between
neurons in neighboring columns with similar preferences
(figure 2.2; Gilbert and Wiesel 1983; Gilbert et al. 1990).
 |
Although the afferent structures and lateral connections are influenced by visual experience during development, after a critical period early in development they are not as easily modified (Movshon and van Sluyters, 1981). However, recent results show that the adult cortex can undergo significant, often reversible, reorganization in response to various sensory and cortical manipulations such as lesions in the receptive surface and the cortex (Gilbert et al., 1996; Gilbert, 1992; Kaas, 1991; Kapadia et al., 1994; Merzenich et al., 1990; Pettet and Gilbert, 1992).