

Next: Oscillatory LFP
Up: The Role of Lateral
Previous: Introduction
The model consists of a cyclic 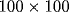 lattice of units
characterized by a voltage variable
lattice of units
characterized by a voltage variable 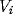 . Each unit models a nerve
cell as a simple RC circuit (
. Each unit models a nerve
cell as a simple RC circuit (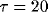 msec) with the addition of a
reset mechanism. Once a unit reaches a threshold voltage
msec) with the addition of a
reset mechanism. Once a unit reaches a threshold voltage 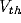 , it
emits a pulse that is transmitted in one iteration (1 msec) to
connected neighboring units, and the potential is reset by subtracting
the voltage threshold. The unit dynamics are
, it
emits a pulse that is transmitted in one iteration (1 msec) to
connected neighboring units, and the potential is reset by subtracting
the voltage threshold. The unit dynamics are
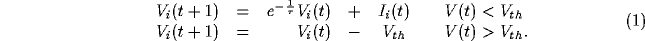
where the current
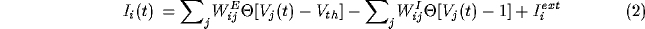
represents the coupling of the unit to nearby unit through excitatory
connections (matrix 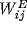 ) and inhibitory connections (matrix
) and inhibitory connections (matrix
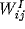 ). Additional input is provided by an independent external
current
). Additional input is provided by an independent external
current 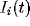 . Such a model, composed of the
``integrate-and-fire'' [24] units with the dynamics
described in eq. 1, is functionally similar to a
recently studied model of self-organized criticality for earthquakes
[38].
. Such a model, composed of the
``integrate-and-fire'' [24] units with the dynamics
described in eq. 1, is functionally similar to a
recently studied model of self-organized criticality for earthquakes
[38].
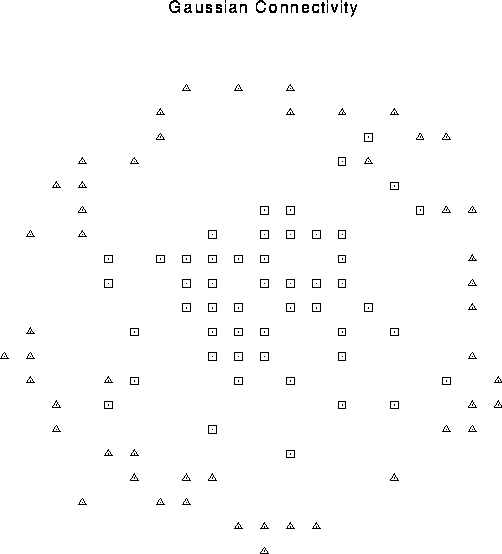
Figure 1:
Center surround connectivity pattern. The cell
(not shown) at the center of the rectangular array is connected in a
probabilistic manner to units within a given distance determined by a
Gaussian distribution with 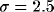 lattice constants. These
short-range connections are excitatory (squares). The center cell
also inhibits a fixed fraction of cells on an annulus 8 and 9 lattice
constants away (triangles). During a particular simulation, the
connectivity pattern is fixed, although the exact synaptic weight
varies stochastically.
lattice constants. These
short-range connections are excitatory (squares). The center cell
also inhibits a fixed fraction of cells on an annulus 8 and 9 lattice
constants away (triangles). During a particular simulation, the
connectivity pattern is fixed, although the exact synaptic weight
varies stochastically.
Based on physiological evidence, the pattern of local excitatory and
inhibitory connections is center-surround, for the validity of
this assumption can be found. Hess and coworkers [19]
found that iontophoretic application of the excitatory agonist
glutamate to visual cortex of anesthetized cats induces excitation of
neurons within 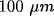 of the application site and distant
inhibition at distances between 100 and
of the application site and distant
inhibition at distances between 100 and 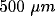 . Similar studies
in rat somatosensory cortical slice preparations
[43] confirm the general pattern of an inhibitory
surround enclosing the excited region. Each unit is excitatorily
connected to N = 50 units chosen from a Gaussian probability
distribution of
. Similar studies
in rat somatosensory cortical slice preparations
[43] confirm the general pattern of an inhibitory
surround enclosing the excited region. Each unit is excitatorily
connected to N = 50 units chosen from a Gaussian probability
distribution of 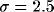 lattice constants, centered at the
unit's position (see Fig. 1). Likewise, N
inhibitory connections per unit are chosen from a uniform probability
distribution on a ring eight to nine lattice constants away. The
weight of the excitation and inhibition, in units of voltage
threshold, is
lattice constants, centered at the
unit's position (see Fig. 1). Likewise, N
inhibitory connections per unit are chosen from a uniform probability
distribution on a ring eight to nine lattice constants away. The
weight of the excitation and inhibition, in units of voltage
threshold, is 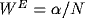 and
and 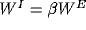 . External
input is modeled independently for each cell as a Poisson process of
excitatory pulses of magnitude
. External
input is modeled independently for each cell as a Poisson process of
excitatory pulses of magnitude 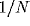 , arriving at a mean rate
, arriving at a mean rate
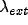 .
.
Scaling the relative degree of inhibition 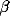 while keeping
the sum of excitation and inhibition constant leads to a
transition from a spatially homogeneous state to a clustered activity state.
This transition can be understood using a mean-field description
of the dynamics, where we write the pulse rate
while keeping
the sum of excitation and inhibition constant leads to a
transition from a spatially homogeneous state to a clustered activity state.
This transition can be understood using a mean-field description
of the dynamics, where we write the pulse rate 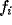 as a function of the
averaged currents
as a function of the
averaged currents 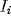 :
:
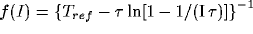 [2],
where
[2],
where 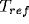 is the minimum dead time between
pulses. In this approximation, the dynamics associated with
eq. 1 simplify to
is the minimum dead time between
pulses. In this approximation, the dynamics associated with
eq. 1 simplify to
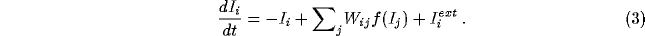
The homogeneous solution is stable only if the connectivity pattern
satisfies 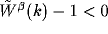 for all k, where
for all k, where
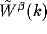 is the Fourier transform of
is the Fourier transform of 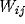 . As
one increases the relative strength of inhibition, clusters of high
firing activity develop. The clusters form a hexagonal grid across
the network, as illustrated in Fig. 2a. Clusters
do not remain stationary but pursue a stochastic motion over the layer
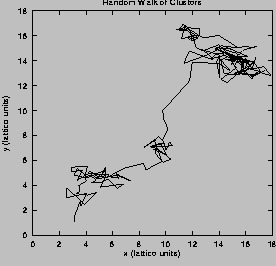
(Fig. 2b).
. As
one increases the relative strength of inhibition, clusters of high
firing activity develop. The clusters form a hexagonal grid across
the network, as illustrated in Fig. 2a. Clusters
do not remain stationary but pursue a stochastic motion over the layer
(Fig. 2b).
Figure 2:
(click on the image to view a larger version)
On the left, the summed activity for the network over 50
msec of simulation is shown for model parameters 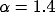 ,
,
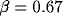 , and
, and 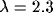 inputs per msec. Lighter shades
denote higher discharge rates (maximum rate 120 Hz). Note the nearly
hexagonal pattern of clusters or ``hot spots" of activity. On the
right, we illustrate the motion of a typical cluster. Each vertex in
the graph represents a tracked cluster's position averaged over 50
msec. The diffusion constant is
inputs per msec. Lighter shades
denote higher discharge rates (maximum rate 120 Hz). Note the nearly
hexagonal pattern of clusters or ``hot spots" of activity. On the
right, we illustrate the motion of a typical cluster. Each vertex in
the graph represents a tracked cluster's position averaged over 50
msec. The diffusion constant is 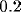 lattice units/msec.
lattice units/msec.
The level of external input also influences the spatial pattern; for
even higher values of input the clusters merge into stripes. The
transition from a homogeneous state to hexagonal clusters to stripes
is generic to many nonequilibrium systems in fluid mechanics,
nonlinear optics, reaction-diffusion systems, and biology
[8,9,12,36].
In the mean-field description, the dynamics (3) are
subject to a Lyapunov potential function [21] so that the
system must relax to a stable state---persistent dynamics are ruled
out.
However, the mean-field equations
[1,52,53] do not
capture the essential dynamics of pulse-coupled networks responsible
for the spatial pattern's metastability. Partial synchronization
inside local populations induce large fluctuations which destabilize
the mean-field pattern. Indeed, simulations performed with constant
(instead of stochastic) input and with isotropic connectivity reveal
that the system is intrinsically chaotic. These fluctuations allow
clusters of high activity to diffuse throughout the system, as can be
seen from the cluster's trajectory in Fig. 2b.
Repulsive interactions with surrounding clusters generally constrain
the motion of a cluster to remain within a certain radius. This
vibratory motion is occasionally punctuated by longer-range diffusion.
If one tracks the activity of individual cells during a period of
time, one observes large fluctuations leading to high variability in
interspike intervals and in the total number of spikes, which are due
to the fact that even under stationary stimulation the system
generates its own rate fluctuations.
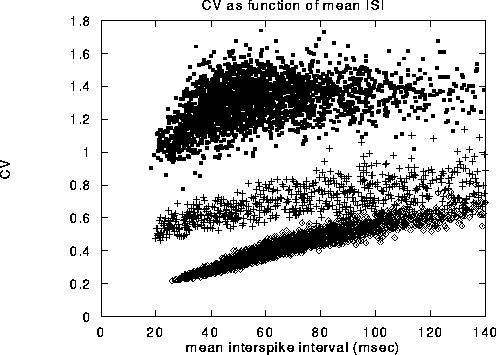
Figure 3:
Coefficient of variation, 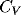 , of a representative range
of cells shown against the average time between spikes, that is, the
inverse of the mean firing rate. The solid dots in the upper cloud are
from our model. The crosses in the middle cloud represent the
behavior when all network effects are eliminated (i.e.
, of a representative range
of cells shown against the average time between spikes, that is, the
inverse of the mean firing rate. The solid dots in the upper cloud are
from our model. The crosses in the middle cloud represent the
behavior when all network effects are eliminated (i.e. 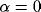 ),
and units receive both an excitatory and an inhibitory stream of
external Poisson input, with
),
and units receive both an excitatory and an inhibitory stream of
external Poisson input, with 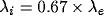 . Without
inhibition, the associated
. Without
inhibition, the associated 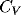 values are reduced by a factor of
about 2. The diamonds in the lowest cloud are from cells in a random
network with sparse, non-local connections that have no organized
topography. The same number of inhibitory and excitatory connections,
50, were used as in the local center-surround connection scheme.
values are reduced by a factor of
about 2. The diamonds in the lowest cloud are from cells in a random
network with sparse, non-local connections that have no organized
topography. The same number of inhibitory and excitatory connections,
50, were used as in the local center-surround connection scheme.
The coefficient of variation (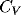 ) computed for all the cells in the
network, over a 400 sec long simulation are displayed in
Fig. 3 Note that almost all values of
) computed for all the cells in the
network, over a 400 sec long simulation are displayed in
Fig. 3 Note that almost all values of 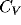 are on the
order of one or larger. The pattern of observed
are on the
order of one or larger. The pattern of observed 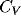 's reproduces
qualitatively the
's reproduces
qualitatively the 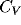 values measured for cells in cortical areas V1
and MT in the awake monkey responding to bars and to clouds of moving
dots [44].
values measured for cells in cortical areas V1
and MT in the awake monkey responding to bars and to clouds of moving
dots [44].
The coefficient of variation does not contain enough information,
however, to fully characterize the statistical properties of the spike
train. In order to obtain a more complete characterization of those
properties, we computed the inter-spike-interval (ISI) distribution
and the cross-correlation function of spike trains
(Fig. 4). We find that the ISI distribution decays as a
power law in t with exponent 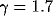 over one decade (from 25
to 300 msec). The correlation
over one decade (from 25
to 300 msec). The correlation 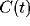 decays slowly via a power law
with exponent
decays slowly via a power law
with exponent 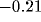 , as shown in Fig. 5. At the upper
temporal cutoff of 300 msec, the correlation function reaches the
baseline of chance coincidence. The power spectrum at low frequencies
has a
, as shown in Fig. 5. At the upper
temporal cutoff of 300 msec, the correlation function reaches the
baseline of chance coincidence. The power spectrum at low frequencies
has a 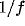 form consistent with the correlation and decays as
form consistent with the correlation and decays as
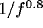 (see, for more details [56]).
(see, for more details [56]).
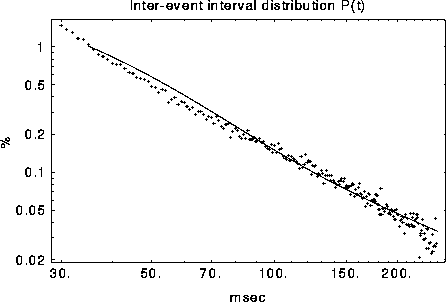
Figure 4:
The average ISI distribution for 19 cells. Superposition of
exponential distributions from the cluster frame of reference (solid
line) results in a power law 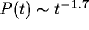 in the fixed unit
frame.
in the fixed unit
frame.
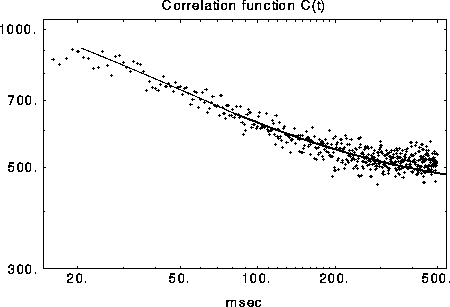
Figure 5:
Measured and predicted (solid line) correlation 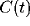 on a
log-log scale. The correlation function decays as
on a
log-log scale. The correlation function decays as 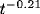 due to
the slow diffusion of activity clusters.
due to
the slow diffusion of activity clusters.
Such power-law distributions of the ISI and correlation functions have
been reported in neurons from the somatosensory cortex [60],
in the auditory pathway [49,50], in the mesencephalic
reticular formation [18] and in visual cortex
[48].
One should note that such power-law functions are not explained by the
variability of spike-trains described by Poisson processes (which have
exponentially distributed ISI) and which are predicted by models based
on a precise balance of the excitatory and the inhibitory inputs to
cortical cells [42,54]. Another
characteristic property of the data which is explained by our
cluster-diffusion model is the typical ``castle-on-hill'' form of
cross-correlations: [33] found cross-correlations
which show a narrow peak 5-50 msec, riding on a much wider, 100-1000
msec, ``hill''. In our model, the narrow peaks result from the
synchronized activity of cells inside a cluster while the wide hills
from the much slower diffusion of the clusters.
A much more direct method to test the prediction of our model is by
means of imaging techniques that tap directly into the spatio-temporal
patterns of neural activity. Using the novel technique of optical
imaging with fast voltage sensitive dyes
[39] report moving elliptical foci of neural
activation in response to electrical stimulation.
Moreover, in a recent study Arieli et al. (1995) [3] have
shown that moving patterns of spontaneous activity on the cortical
surface contribute to the variability of neural response.


Next: Oscillatory LFP
Up: The Role of Lateral
Previous: Introduction
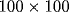 lattice of units
characterized by a voltage variable
lattice of units
characterized by a voltage variable 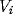 . Each unit models a nerve
cell as a simple RC circuit (
. Each unit models a nerve
cell as a simple RC circuit (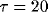 msec) with the addition of a
reset mechanism. Once a unit reaches a threshold voltage
msec) with the addition of a
reset mechanism. Once a unit reaches a threshold voltage 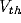 , it
emits a pulse that is transmitted in one iteration (1 msec) to
connected neighboring units, and the potential is reset by subtracting
the voltage threshold. The unit dynamics are
, it
emits a pulse that is transmitted in one iteration (1 msec) to
connected neighboring units, and the potential is reset by subtracting
the voltage threshold. The unit dynamics are
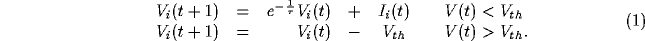
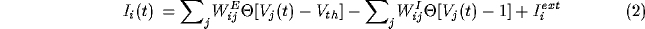
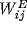 ) and inhibitory connections (matrix
) and inhibitory connections (matrix
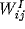 ). Additional input is provided by an independent external
current
). Additional input is provided by an independent external
current 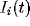 . Such a model, composed of the
``integrate-and-fire'' [
. Such a model, composed of the
``integrate-and-fire'' [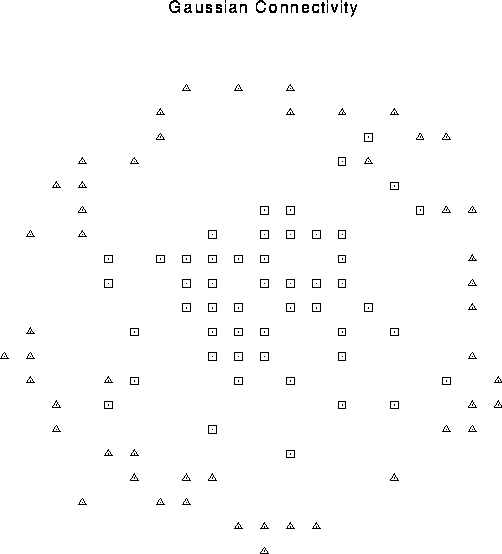
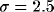 lattice constants. These
short-range connections are excitatory (squares). The center cell
also inhibits a fixed fraction of cells on an annulus 8 and 9 lattice
constants away (triangles). During a particular simulation, the
connectivity pattern is fixed, although the exact synaptic weight
varies stochastically.
lattice constants. These
short-range connections are excitatory (squares). The center cell
also inhibits a fixed fraction of cells on an annulus 8 and 9 lattice
constants away (triangles). During a particular simulation, the
connectivity pattern is fixed, although the exact synaptic weight
varies stochastically. 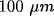 of the application site and distant
inhibition at distances between 100 and
of the application site and distant
inhibition at distances between 100 and 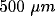 . Similar studies
in rat somatosensory cortical slice preparations
[
. Similar studies
in rat somatosensory cortical slice preparations
[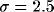 lattice constants, centered at the
unit's position (see Fig.
lattice constants, centered at the
unit's position (see Fig. 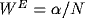 and
and 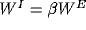 . External
input is modeled independently for each cell as a Poisson process of
excitatory pulses of magnitude
. External
input is modeled independently for each cell as a Poisson process of
excitatory pulses of magnitude 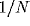 , arriving at a mean rate
, arriving at a mean rate
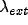 .
.
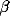 while keeping
the sum of excitation and inhibition constant leads to a
transition from a spatially homogeneous state to a clustered activity state.
This transition can be understood using a mean-field description
of the dynamics, where we write the pulse rate
while keeping
the sum of excitation and inhibition constant leads to a
transition from a spatially homogeneous state to a clustered activity state.
This transition can be understood using a mean-field description
of the dynamics, where we write the pulse rate 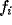 as a function of the
averaged currents
as a function of the
averaged currents 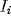 :
:
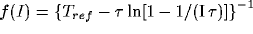 [
[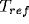 is the minimum dead time between
pulses. In this approximation, the dynamics associated with
eq.
is the minimum dead time between
pulses. In this approximation, the dynamics associated with
eq. 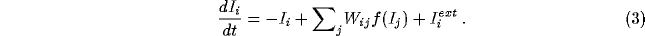
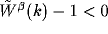 for all k, where
for all k, where
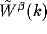 is the Fourier transform of
is the Fourier transform of 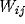 . As
one increases the relative strength of inhibition, clusters of high
firing activity develop. The clusters form a hexagonal grid across
the network, as illustrated in Fig.
. As
one increases the relative strength of inhibition, clusters of high
firing activity develop. The clusters form a hexagonal grid across
the network, as illustrated in Fig. 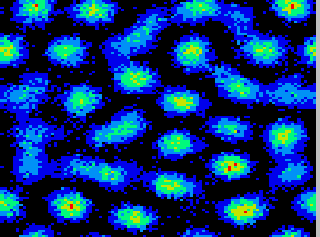
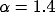 ,
,
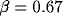 , and
, and 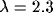 inputs per msec. Lighter shades
denote higher discharge rates (maximum rate 120 Hz). Note the nearly
hexagonal pattern of clusters or ``hot spots" of activity. On the
right, we illustrate the motion of a typical cluster. Each vertex in
the graph represents a tracked cluster's position averaged over 50
msec. The diffusion constant is
inputs per msec. Lighter shades
denote higher discharge rates (maximum rate 120 Hz). Note the nearly
hexagonal pattern of clusters or ``hot spots" of activity. On the
right, we illustrate the motion of a typical cluster. Each vertex in
the graph represents a tracked cluster's position averaged over 50
msec. The diffusion constant is 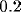 lattice units/msec.
lattice units/msec.
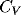 , of a representative range
of cells shown against the average time between spikes, that is, the
inverse of the mean firing rate. The solid dots in the upper cloud are
from our model. The crosses in the middle cloud represent the
behavior when all network effects are eliminated (i.e.
, of a representative range
of cells shown against the average time between spikes, that is, the
inverse of the mean firing rate. The solid dots in the upper cloud are
from our model. The crosses in the middle cloud represent the
behavior when all network effects are eliminated (i.e. 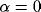 ),
and units receive both an excitatory and an inhibitory stream of
external Poisson input, with
),
and units receive both an excitatory and an inhibitory stream of
external Poisson input, with 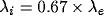 . Without
inhibition, the associated
. Without
inhibition, the associated 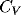 values are reduced by a factor of
about 2. The diamonds in the lowest cloud are from cells in a random
network with sparse, non-local connections that have no organized
topography. The same number of inhibitory and excitatory connections,
50, were used as in the local center-surround connection scheme.
values are reduced by a factor of
about 2. The diamonds in the lowest cloud are from cells in a random
network with sparse, non-local connections that have no organized
topography. The same number of inhibitory and excitatory connections,
50, were used as in the local center-surround connection scheme. 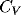 ) computed for all the cells in the
network, over a 400 sec long simulation are displayed in
Fig.
) computed for all the cells in the
network, over a 400 sec long simulation are displayed in
Fig. 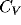 are on the
order of one or larger. The pattern of observed
are on the
order of one or larger. The pattern of observed 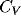 's reproduces
qualitatively the
's reproduces
qualitatively the 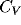 values measured for cells in cortical areas V1
and MT in the awake monkey responding to bars and to clouds of moving
dots [
values measured for cells in cortical areas V1
and MT in the awake monkey responding to bars and to clouds of moving
dots [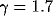 over one decade (from 25
to 300 msec). The correlation
over one decade (from 25
to 300 msec). The correlation 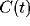 decays slowly via a power law
with exponent
decays slowly via a power law
with exponent 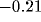 , as shown in Fig.
, as shown in Fig. 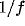 form consistent with the correlation and decays as
form consistent with the correlation and decays as
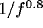 (see, for more details [
(see, for more details [
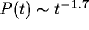 in the fixed unit
frame.
in the fixed unit
frame. 
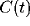 on a
log-log scale. The correlation function decays as
on a
log-log scale. The correlation function decays as 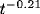 due to
the slow diffusion of activity clusters.
due to
the slow diffusion of activity clusters.