

Next: Discussion and Future Work
Up: A Neural Network Model
Previous: Architecture
Subsections
The model consisted of an array of 192 × 192 neurons, and a
retina of 24 × 24 ganglion cells. The circular anatomical
receptive field of each neuron was centered in the portion of the
retina corresponding to the location of the neuron in the cortex. The
RF consisted of random-strength connections to all ganglion cells less
than 6 units away from the RF center.
The cortex was self-organized for 30,000 iterations on oriented
Gaussian inputs with major and minor axes of half-width 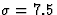 and 1.5 , respectively.
and 1.5 , respectively.![[*]](foot_motif.gif) The training took 8 hours on 64 processors of a Cray T3D at the
Pittsburgh Supercomputing Center. The model requires more than
three gigabytes of physical memory to represent the more than 400
million connections in this small section of the cortex.
The training took 8 hours on 64 processors of a Cray T3D at the
Pittsburgh Supercomputing Center. The model requires more than
three gigabytes of physical memory to represent the more than 400
million connections in this small section of the cortex.
In the self-organization process, the neurons developed oriented
receptive fields organized into orientation columns very similar to
those observed in the primary visual cortex.
The strongest lateral connections of highly-tuned cells
link areas of similar orientation preference, and avoid neurons with
the orthogonal orientation preference.
Furthermore, the connection patterns of highly oriented neurons are
typically elongated along the direction in the map that corresponds to
the neuron's preferred stimulus orientation. This organization
reflects the activity correlations caused by the elongated Gaussian
input pattern: such a stimulus activates primarily those neurons that
are tuned to the same orientation as the stimulus, and located along
its length (Sirosh, Miikkulainen, and Bednar, 1996).
Since the long-range lateral connections are inhibitory, the net
result is decorrelation: redundant activation is removed,
resulting in a sparse representation of the novel features of each
input (Barlow 1990; Field 1994;
Sirosh, Miikkulainen, and Bednar 1996).
As a side effect, illusions and aftereffects may sometimes occur, as
will be shown below.
In psychophysical measurements of the TAE, a fixed stimulus is
presented at a particular location on the retina. To simulate these
conditions in the model, the position and angle of the inputs were
fixed to a single value for a number of iterations, rather than having
a uniform random distribution as in self-organization. To permit more
detailed analysis of behavior at short time scales, the learning rates
were reduced from those used during self-organization, to
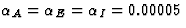 . All other parameters remained
as in self-organization.
. All other parameters remained
as in self-organization.
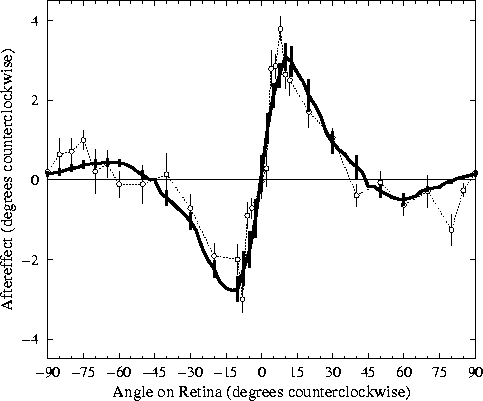
Figure 3:
Tilt aftereffect versus retinal angle.
The open circles represent the average tilt aftereffect for a
single human subject (DEM) from
Mitchell and Muir (1976) over ten
trials. For each angle in each trial, the subject adapted for
three minutes on a sinusoidal grating of a given angle, then was
tested for the effect on a horizontal grating. Error bars indicate
±1 standard error of measurement. The subject shown had the
most complete data of the four in the study. All four showed very
similar effects in the x-axis range ±40°; the
indirect TAE for the larger angles varied widely between
±2.5°. The graph is roughly anti-symmetric around
0°, so the TAE is essentially the same in both directions
relative to the adaptation line.
The heavy line shows the average magnitude of the tilt aftereffect
in the RF-LISSOM model over nine trials at different locations on
the retina. Error bars indicate ±1 standard error of
measurement. The network adapted to a vertical adaptation line at
a particular position for 90 iterations, then the TAE was measured
for test lines oriented at each angle. The duration of adaptation
was chosen so that the magnitude of the human data and the model
match; this was the only parameter fit to the data.
The result from the model
closely resembles the curve for humans at all angles, showing both
direct and indirect tilt aftereffects.
 |
To compare with the psychophysical experiments, perceived
orientations were compared before and after tilt adaptation.
Perceived orientation was measured as a vector sum over all active
neurons, with the magnitude of each vector representing the activation
level, and the vector direction representing the orientation
preference of the neuron before adaptation. Perceived orientation was
computed separately for each possible orientation of the test
Gaussian, both before and after adaptation. For a given angular
separation of the adaptation stimulus and the test stimulus, the
computed magnitude of the tilt aftereffect is the difference between
the initial perceived angle and the one perceived after adaptation.
Figure 3 plots these differences after adaptation
for 90 iterations of the RF-LISSOM algorithm. For comparison,
figure 3 also shows the most detailed data
available for the TAE in human foveal vision (Mitchell and Muir, 1976).
The results from the RF-LISSOM simulation are strikingly similar to
the psychophysical results. For the range 5° to 40°, all
subjects in the human study (including the one shown) exhibited angle
repulsion effects nearly identical to those found in the RF-LISSOM
model. The magnitude of this direct TAE increases very rapidly
to a maximum angle repulsion at approximately 10°, falling off
somewhat more gradually to zero as the angular separation increases.
The results for larger angular separations (from 45° to 85°)
show a greater inter-subject variability in the psychophysical
literature, but those found for the RF-LISSOM model are well within
the range seen for human subjects. The indirect effects for
the subject shown were typical for that study, although some subjects
showed effects up to 2.5°.
In addition to the angular changes in the TAE, its magnitude in humans
increases regularly with adaptation time (Gibson and Radner, 1937).
The equivalent of ``time'' in the RF-LISSOM model is an iteration,
i.e. a single cycle of input presentation, activity propagation,
settling, and weight modification. As the number of adaptation
iterations is increased, the magnitude of the TAE in the model
increases monotonically, while retaining the same basic shape of
figure 3 (Bednar, 1997). The curve
that best matches the human data was shown in
figure 3.
Due to the time required to obtain even a single point on the angular
curve of the TAE for human subjects, complete experimental
measurements of the angular function at different adaptation times are
not available. However, when the time course of the direct TAE is
measured at a single orientation, the increase is approximately
logarithmic with time (Gibson and Radner, 1937), eventually saturating
at a level that depends upon the experimental protocol used
(Greenlee and Magnussen, 1987; Magnussen and Johnsen, 1986).
Figure 4 compares the shape of this TAE
versus time curve for human subjects and for the RF-LISSOM model.
Figure 4:
Direct tilt aftereffect versus time.
The circles show the magnitude of the TAE as a function of
adaptation time for human subjects MWG (unfilled circles) and SM
(filled circles) from
Greenlee and Magnussen (1987); they were the
only subjects tested in the study. Each subject adapted to a
single +12°line for the time period indicated on the
horizontal axis (bottom). To estimate the magnitude of the
aftereffect at each point, a vertical test line was presented at
the same location and the subject was requested to set a
comparison line at another location to match it. The plots
represent averages of five runs; the data for 0 - 10 minutes were
collected separately from the rest.
For comparison, the heavy line shows average TAE in the LISSOM
model for a +12°test line over 9 trials (with parameters as in
figure
3). The horizontal axis (top)
represents the number of iterations of adaptation, and the
vertical axis represents the magnitude of the TAE at this time
step.
The RF-LISSOM results show a similar logarithmic increase in TAE
magnitude with time, but do not show the saturation that is seen
for the human subjects.
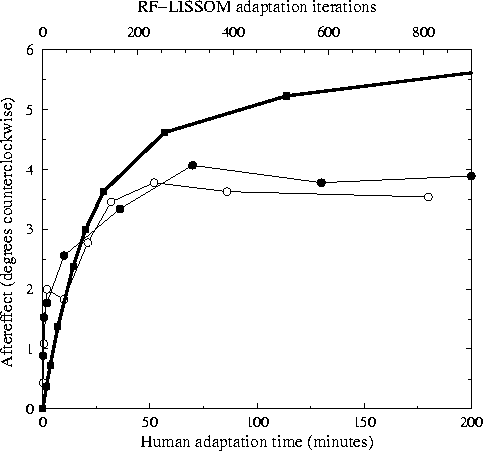 |
The x axis for the RF-LISSOM and human data has different units, but
the correspondence between the two curves might provide a rough way of
quantifying the equivalent real time for an ``iteration'' of the
model. The time course of the TAE in the RF-LISSOM model is similar
to the human data. The TAE increases approximately logarithmically,
but it does not completely saturate over the adaptation amounts tested
so far. This difference suggests that the biological implementation
has additional constraints on the amount of learning that can be
achieved over the time scale over which the tilt aftereffect is seen.
The TAE seen in figures 3
and 4 must result from changes
in the connection strengths between neurons, since no other
component of the model changes as adaptation progresses.
Simulations performed with only one type of weight (either afferent,
lateral excitatory, or lateral inhibitory) adapting at a given time
show that the inhibitory weights determine the shape of the curve for
all angles (Bednar, 1997). The small component of the TAE
resulting from adaptation of either type of excitatory weights is
almost precisely opposite the total effect.
Although each inhibitory connection adapts with the same learning rate
as the excitatory connections (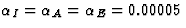 ),
there are many more inhibitory connections than excitatory
connections.
The combined strength of all the small inhibitory changes outweighs
the excitatory changes, and results in a curve with a sign opposite
that of the components from the excitatory weights.
),
there are many more inhibitory connections than excitatory
connections.
The combined strength of all the small inhibitory changes outweighs
the excitatory changes, and results in a curve with a sign opposite
that of the components from the excitatory weights.
In what way do the changing inhibitory connections cause these
effects? During adaptation, we see that the response to the 0°
adaptation line becomes gradually more concentrated towards the
central area of the Gaussian pattern presented. This is because the
inhibition between active neurons increases, allowing only the most
strongly activated neurons to remain active after settling
(equation 2). However, the distribution of active
orientation detectors is centered around the same angle, so
the same angle is perceived.
The response to a test line with a slightly different orientation
(e.g. 10°) is also more focused after adaptation, but the overall
distribution of activated neurons has shifted. Fewer neurons that
prefer orientations close to the adaptation line now respond, but an
increased number of those that prefer distant angles do. This is
because inhibition was strengthened primarily between neurons close to
the adaptation angle, and not between those which prefer larger
orientations, greater than the 10° test line. The net effect is a
shift of the perceived orientation away from the adaptation
angle, resulting in the direct TAE.
In contrast, the response to a very different test line (e.g. 60°)
is broader and stronger after adaptation. Adaptation occurred only in
activated neurons, so neurons with orientation preferences greater
than 60°are unchanged. However, those with preferences somewhat
less than 60° actually now respond more strongly. During
adaptation, their inhibitory connections with other active neurons,
i.e. those that represent orientations close to the 0° adaptation line, became
stronger. Since the sum of inhibition is constant for each neuron
(equation 3), the connections to neurons representing
distant angles (e.g. 60°) became weaker. As a result, the 60°
line now inhibits them less than before adaptation. Thus they are
more active, and the perceived orientation has shifted towards 0°.
This indirect effect is therefore true to its name, caused indirectly
by the strengthening of inhibitory connections. The RF-LISSOM model
thus shows computationally that both the direct and indirect effects
could be caused by activity-dependent adaptation of inhibitory lateral
interactions.


Next: Discussion and Future Work
Up: A Neural Network Model
Previous: Architecture
James A. Bednar
9/15/1997
![[*]](foot_motif.gif) The training took 8 hours on 64 processors of a Cray T3D at the
Pittsburgh Supercomputing Center. The model requires more than
three gigabytes of physical memory to represent the more than 400
million connections in this small section of the cortex.
The training took 8 hours on 64 processors of a Cray T3D at the
Pittsburgh Supercomputing Center. The model requires more than
three gigabytes of physical memory to represent the more than 400
million connections in this small section of the cortex.

