

Next: 4 Discussion and Future
Up: Tilt Aftereffects in a
Previous: 2 Architecture
Subsections
The model consisted of an array of 192 × 192 neurons, and a
retina of 36 × 36 ganglion cells. The center of the
anatomical receptive field of each neuron was placed at the location
in the central 24 × 24 portion of the retina corresponding to the
location of the neuron in the cortex, so that every neuron would have a
complete set of afferent connections. The RF had a circular shape,
consisting of random-strength connections to all ganglion cells within
6 units from the RF center.
The cortex was self-organized for 20,000 iterations on oriented
Gaussian inputs with major and minor axes of half-width 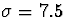 and 1.5 , respectively.
and 1.5 , respectively.![[*]](foot_motif.gif) The training took 2.5 hours on 16 processors of a Cray T3E
supercomputer at the Texas Advanced Computing Center. The model
requires 1.5 gigabytes of physical memory to represent the 400 million
connections in this small section of the cortex.
The training took 2.5 hours on 16 processors of a Cray T3E
supercomputer at the Texas Advanced Computing Center. The model
requires 1.5 gigabytes of physical memory to represent the 400 million
connections in this small section of the cortex.
In the self-organization process, the neurons developed oriented
receptive fields organized into orientation columns very similar to
those observed in the primary visual cortex (figure 3b).
Figure 3:
Orientation map activation.
The orientation color key underneath (
b ) applies
to all of the graphs in (
b-d ). After
being trained on inputs like the one in (
a ) with random
positions and orientations, the RF-LISSOM network developed
the orientation map shown in (
b ). Each neuron is colored according to the
orientation it prefers.
The black outline shows the extent of the
patchy self-organized lateral inhibitory connections of one
neuron (marked with a black square) which has a
vertical orientation preference. The strongest connections of
each neuron are extended along its preferred orientation and link
columns with similar orientation preferences, avoiding those with
orthogonal preferences.
The brightness of the colors in (
c,d ) shows
the strength of activation for each neuron to pattern
(
a ). The initial response of the organized map is spatially
broad and diffuse (
c, top ), like the input, and its
cortical location at, above, and below the center of the cortex
indicates that the input is vertically extended around the center
of the retina. The
response is patchy because the network is also encoding
orientation, and the neurons that encode orientations far from the
vertical do not respond (compare
c to
b ). The
histogram (
c, bottom ) sums up the orientation coding of
the response. Each bin represents a range of 5°, which is
the precision to which the orientation map was measured. A wide
range of neurons preferring orientations around 0° are
activated, but the average orientation is approximately 0°
(-0.6° for this particular run). After the network
settles through lateral interactions, the activation is much more
focused, both spatially (
d, top ) and in representing
orientation (
d, bottom ), but the spatial and orientation
averages continue to match the position and orientation of the
input, respectively. The average orientation of the settled
response (-1.3° here) is taken to be the perceived
orientation for the TAE experiments. Animated demos of these figures can be seen at
http://www.cs.utexas.edu/users/nn/pages/research/selforg.html
.
![\begin{figure}
\centering
\begin{minipage}
{\textwidth}\begin{minipage}[t]
{\fo...
...emph{d}\/) Settled activity\end{center}\end{minipage}\end{minipage} \end{figure}](img29.gif) |
The strongest lateral connections of highly-tuned cells
link areas of similar orientation preference, and avoid neurons with
the orthogonal orientation preference.
Furthermore, the connection patterns of highly oriented neurons are
typically elongated along the direction in the map that corresponds to
the neuron's preferred stimulus orientation (as subsequently found
experimentally by Bosking et al. 1997.) This organization reflects
the activity correlations caused by the elongated Gaussian input
pattern: such a stimulus activates primarily those neurons that are
tuned to the same orientation as the stimulus, and located along its
length (Sirosh et al., 1996).
Since the long-range lateral connections are inhibitory, the net
result is decorrelation : redundant activation is removed,
resulting in a sparse representation of the novel features of each
input (Barlow, 1990; Field, 1994; Sirosh et al., 1996).
As a side effect, illusions and aftereffects may sometimes occur, as
will be shown below.
In psychophysical measurements of the TAE, a fixed stimulus is
presented at a particular location on the retina. To simulate these
conditions in the RF-LISSOM model, the position and angle of the
inputs were fixed to a single value for a number of iterations. To
permit more detailed analysis of behavior at short time scales, the
learning rates were reduced from those used during self-organization
to 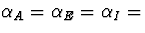 0.000005. All other parameters
remained as in self-organization.
0.000005. All other parameters
remained as in self-organization.
To compare with the psychophysical experiments, it is necessary to
determine what orientation the model ``perceives'' for any given
input. Precisely how neural responses are interpreted for perception
remains quite controversial (see Parker and Newsome 1998 for review), but
results in primates suggest that behavioral performance approaches the
statistical optimum given the measured properties of cortical neurons
(Geisler and Albrecht, 1997). Accordingly, we extracted the perceived
orientation using a vector sum procedure, which has been shown to be
optimal under conditions present in the model (Snippe, 1996).
For this procedure, each active neuron was represented by a vector
whose magnitude corresponded to the activation level and whose
direction corresponded to the orientation preference of the neuron.
Perceived orientation was then measured as a vector sum over all
neurons that responded to the input. In the model, the perceived
orientation was found to match the absolute orientation of the input
pattern to within a few degrees (Bednar, 1997).
To determine the tilt aftereffect in the model, the perceived
orientation was first computed for inputs of all orientations. The
model then adapted to a fixed input for 90 iterations, and the
perceived orientation was again computed for all inputs. The
difference between the initial perceived angle and the one perceived
after adaptation was taken as the magnitude of the TAE.
Figure 4 plots these differences for the different
angles. For comparison, figure 4 also shows the
most detailed data available for the TAE in human foveal vision
(Mitchell and Muir, 1976).
Figure 4:
Tilt aftereffect at different angles.
The open circles represent the tilt aftereffect for a
single human subject (DEM) from
Mitchell and Muir (1976) averaged over ten
trials. For each angle in each trial, the subject adapted for
three minutes on a sinusoidal grating of a given angle, then was
tested for the effect on a horizontal grating. Error bars indicate
±1 standard error of measurement. The subject shown had the
most complete data of the four in the study. All four showed very
similar effects in the
x -axis range ±40°; the
indirect TAE for the larger angles varied widely between
±2.5°. The graph is roughly anti-symmetric around
0°, so the TAE is essentially the same in both directions
relative to the adaptation line.
For comparison, the heavy line shows the average magnitude of the
tilt aftereffect in the RF-LISSOM model over ten trials with
different adaptation angles. Error bars indicate ±1 standard
error of measurement; in most cases they are too small to be
visible since the results were very consistent between different
runs. The network adapted to an oriented line at the center of
the retina for 90 iterations, then the TAE was measured for test
lines oriented at each angle. The duration of adaptation was
chosen so that the magnitude of the human data and the model
match; this was the only parameter fit to the data.
The result from the model closely resembles the curve for humans,
showing both direct and indirect tilt aftereffects.
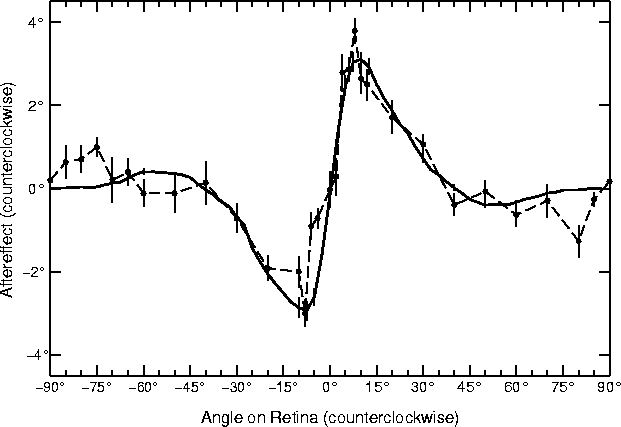 |
The results from the RF-LISSOM simulation are strikingly similar to
the psychophysical results. For the range 5° to 40°, all
subjects in the human study exhibited angle repulsion effects nearly
identical to those found in the RF-LISSOM model; the data was most
complete for the subject shown. The magnitude of this direct TAE
increases very rapidly to a maximum angle repulsion at 8-10°,
falling off somewhat more gradually to zero as the angular separation
increases. The simulations with larger angular separations (from
40° to 85°) show a smaller angle attraction, i.e. the
indirect effect. Although there is a greater inter-subject
variability in the psychophysical literature for the indirect effect
than the direct effect, those found for the RF-LISSOM model are well
within the range seen for human subjects.
In parallel with the angular changes in the TAE, its peak magnitude in
humans increases logarithmically with adaptation time
(Gibson and Radner, 1937), eventually saturating at a level that
depends upon the experimental protocol used
(Greenlee and Magnussen, 1987; Magnussen and Johnsen, 1986). As the number of
adaptation iterations is increased in the model, the magnitude of the
TAE also increases logarithmically
(figure 5). (The single curve that best
matched the magnitude of the human data was shown in
figure 4, but the ones for different amounts of
adaptation all had the same basic shape.) The time course of the TAE
in the RF-LISSOM model is qualitatively similar to the human data, but
it does not completely saturate over the adaptation amounts tested so
far.
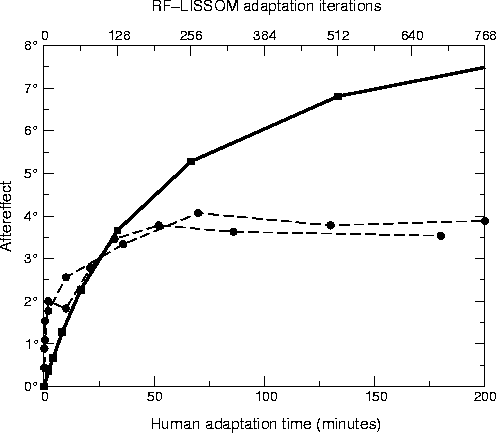
Figure 5:
Direct TAE over time.
The circles show the magnitude of the TAE as a function of
adaptation time for human subjects MWG (unfilled circles) and SM
(filled circles) from
Greenlee and Magnussen (1987); they were the
only subjects tested in the study. Each subject adapted to a
single +12° line for the time period indicated on the
horizontal axis (bottom). To estimate the magnitude of the
aftereffect at each point, a vertical test line was presented at
the same location and the subject was requested to set a
comparison line at another location to match it. The plots
represent averages of five runs; the data for 0 - 10 minutes were
collected separately from the rest.
For comparison, the heavy line shows average TAE in the LISSOM
model for a +12° test line over 9 trials (with
parameters as in figure
4). The horizontal
axis (top) represents the number of iterations of adaptation, and
the vertical axis represents the magnitude of the TAE at this time
step.
The RF-LISSOM results show a similar logarithmic increase in TAE
magnitude with time, but do not show the saturation that is seen
for the human subjects.
 |
The TAE seen in figure 4
must result from changes in the connection strengths between neurons,
since no other component of the model changes as adaptation
progresses. Simulations performed with only one type of weight
adapting (either afferent, lateral excitatory, or lateral inhibitory)
show that the inhibitory weight changes determine the shape of
the curve for all angles (Bednar, 1997).
In what way do the changing inhibitory connections cause these
effects?
We will demonstrate this process in a simulation where only inhibitory
weights adapted, the adaptation period was longer, and a higher
learning rate was used, all in order to exaggerate the effect and make
its causes more clearly visible. First of all, the connections change
in a way that leaves the perceived orientation of the adaptation line
unchanged. Figure 6a
Figure 6:
Explanation of the TAE.
This figure shows activation histograms similar to those in
figure
3c
and
3d for several test lines.
The histograms focus on the orientation-specific aspects of the
cortical response by abstracting out the spatial content, and
demonstrate how changes in the response cause the TAE.
The top row of histograms (marked
I, for Initial)
shows the initial response to a vertical line (0°), a
10° line, and a 50° line, each marked by dotted
lines. In each case, the initial response is roughly centered
around the orientation of the input line. The next row (marked
S, Settled) shows the settled response, which has
been focused by the lateral connections but is still centered
around the input orientation. The bottom row (marked
A, Adapted) shows the settled response to the same
input
after adapting to a vertical (0°) line, marked
by a vertical line on the plots. To magnify and clarify the
effect for explanatory purposes, only the inhibitory weights were
modifiable in this simulation, their learning rate was increased
to 0.00005, and the adaptation lasted for 256 iterations.
In (
a ), the settled response to the 0° line broadens
with adaptation, as the inhibition between the active units
increases (
aS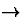 A
A ).
Since the response remains centered around 0°, there is
little change in the perceived orientation and the TAE is close to
0° (as can also be seen in figure
4).
In contrast in (
b ), a dramatic orientation shift is
evident: while the settled histogram before adaptation was
centered around 7.9°, after adaptation it is centered around
20.3°
(
bS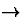 A
A ), inducing a
direct effect of 12.4°. The direct TAE is caused by the
same changes that caused the broadening in (
a ): the
activity around 0° has decreased, while the activity at
larger angles has increased.
The changes are more subtle for the indirect effect (
c ).
For the 50° stimulus, only the neurons around
0° in (
cI ) fall in the range of
orientations initially activated by the adaptation line
(
aI ), and thus those are the only ones that
change their behavior between (
cS ) and
(
cA ). During adaptation, their inhibition
to and from neurons around 0° was increased, and the weight
normalization caused a corresponding decrease to other neurons,
including those around 50°. As a result, they are now less
inhibited than before adaptation, and the average response shifts
towards the adaptation angle (the indirect TAE).
Animated demos
of these examples can be seen at
http://www.cs.utexas.edu/users/nn/pages/research/selforg.html
.
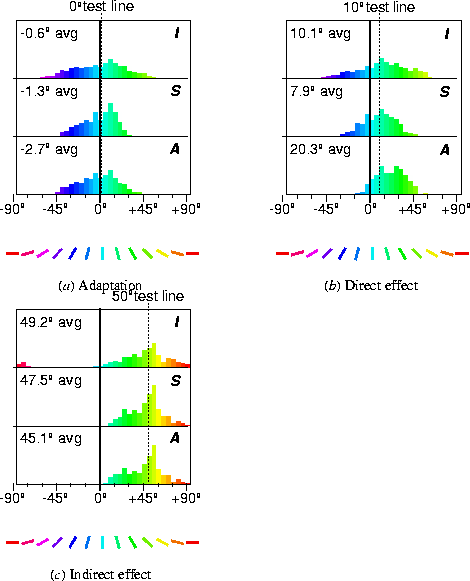 |
shows the initial response (I), the settled response
(S), and the settled response after adaptation
(A) for a vertical input (0°, marked with a
vertical line.) The response after adaptation is more diffuse because
the most active neurons have become inhibited (equation 3).
Throughout adaptation, the distribution of active orientation
detectors is centered around approximately the same angle, and a
constant angle is perceived.
The initial and settled responses to a test line with a slightly
different orientation (e.g. 10°, marked with a dotted line in
figure 6b ) are again centered around that
orientation (6b I and
S). However, comparing the histograms of settled
activity before and after adaptation
(6b S and A), it
is clear that fewer neurons close to 0° respond after
adaptation, but an increased number of those representing distant
angles (over 10°) do. This is because during adaptation,
inhibition was strengthened primarily between neurons close to the
0° adaptation angle, and not between those that prefer larger
orientations.
Such adaptation increases the ability of
the map to detect small differences from the adaptation line. Before
adaptation, the settled histograms for 0° and 10° are
fairly similar, with averages differing by only
9.2°
in this simulation
(6a S
and 6b S). After
adaptation, the histograms are very different, resulting in
a 23°
difference
in perceived orientation
(compare 6a A
with 6b A). This adaptation is
manifested at the psychological level as a shift of the perceived
orientation away from the adaptation line, that is, the direct
TAE.
Meanwhile, the response to a very different test line (e.g.
50°, the dotted line in figure 6c )
becomes broader and stronger after adaptation
(compare 6c S and A).
Adaptation occurred only in activated neurons, so neurons with
orientation preferences greater than 50° are unchanged.
However, those with preferences less than 50° actually now
respond more strongly
(6c S and A). The
reason is that during
adaptation, the inhibitory connections of these neurons with each
other became stronger. Because of normalization
(equation 3), their connections to other neurons, i.e.
those representing distant angles such as 50°, became weaker.
As a result, the 50° line now inhibits them less than before
adaptation. Thus they are more active, and the perceived orientation
shifts towards 0°, causing the indirect tilt aftereffect.
The indirect effect is therefore true to its name, caused indirectly
by the strengthening of inhibitory connections during adaptation.
This explanation of the indirect effect is novel, and emerges
automatically from the RF-LISSOM model. The model thus shows
computationally how both the direct and indirect effects can be caused
by the same activity-dependent adaptation process, and that it is the
same process that drives the development of the map.
Footnotes
- ...respectively.
-
The initial lateral excitation
radius was 19 and was gradually decreased to 1 . The lateral
inhibitory radius of each neuron was 47 , and inhibitory
connections whose strength was below 0.00005 were pruned away at
20,000 iterations. The lateral inhibitory connections were initialized
to a Gaussian profile with
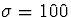 , and the lateral excitatory
connections to a Gaussian with
, and the lateral excitatory
connections to a Gaussian with 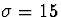 , with no connections
outside the nominal circular radius. The lateral excitation
, with no connections
outside the nominal circular radius. The lateral excitation
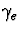 and inhibition strength
and inhibition strength 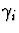 were both 0.9 . The
learning rate
were both 0.9 . The
learning rate 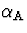 was gradually decreased from 0.007
to 0.0015 ,
was gradually decreased from 0.007
to 0.0015 , 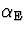 from 0.002 to 0.001 and
from 0.002 to 0.001 and
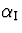 was a constant 0.00025 . The lower and upper
thresholds of the sigmoid were increased from 0.1 to 0.24 and
from 0.65 to 0.88 , respectively. The number of iterations for
which the lateral connections were allowed to settle at each
training iteration was initially 9 , and was increased to 13 over
the course of training. These parameter settings were used
by Sirosh et al. to model development of the orientation map, and
were not tuned or tweaked for the tilt aftereffect simulations.
Small variations produce roughly equivalent results
(Sirosh, 1995).
was a constant 0.00025 . The lower and upper
thresholds of the sigmoid were increased from 0.1 to 0.24 and
from 0.65 to 0.88 , respectively. The number of iterations for
which the lateral connections were allowed to settle at each
training iteration was initially 9 , and was increased to 13 over
the course of training. These parameter settings were used
by Sirosh et al. to model development of the orientation map, and
were not tuned or tweaked for the tilt aftereffect simulations.
Small variations produce roughly equivalent results
(Sirosh, 1995).


Next: 4 Discussion and Future
Up: Tilt Aftereffects in a
Previous: 2 Architecture
James A. Bednar
8/2/1999
![[*]](foot_motif.gif) The training took 2.5 hours on 16 processors of a Cray T3E
supercomputer at the Texas Advanced Computing Center. The model
requires 1.5 gigabytes of physical memory to represent the 400 million
connections in this small section of the cortex.
The training took 2.5 hours on 16 processors of a Cray T3E
supercomputer at the Texas Advanced Computing Center. The model
requires 1.5 gigabytes of physical memory to represent the 400 million
connections in this small section of the cortex.
![]() and 1.5 , respectively.
and 1.5 , respectively.![[*]](foot_motif.gif) The training took 2.5 hours on 16 processors of a Cray T3E
supercomputer at the Texas Advanced Computing Center. The model
requires 1.5 gigabytes of physical memory to represent the 400 million
connections in this small section of the cortex.
The training took 2.5 hours on 16 processors of a Cray T3E
supercomputer at the Texas Advanced Computing Center. The model
requires 1.5 gigabytes of physical memory to represent the 400 million
connections in this small section of the cortex.
![\begin{figure}
\centering
\begin{minipage}
{\textwidth}\begin{minipage}[t]
{\fo...
...emph{d}\/) Settled activity\end{center}\end{minipage}\end{minipage} \end{figure}](img29.gif)
![]() 0.000005. All other parameters
remained as in self-organization.
0.000005. All other parameters
remained as in self-organization.


